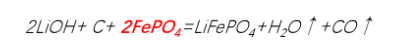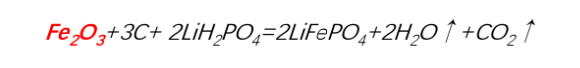Lithium iron phosphate has an olivine structure and is characterized by good cycle performance, stable electrochemical properties, and low cost. It is commonly used as the cathode material for power lithium-ion batteries.
The molecular formula of lithium iron phosphate is LiFePO4. Its principle of action during the charging and discharging processes of lithium batteries is as follows:
When a lithium battery is being charged, lithium ions Li+ detach from the lithium iron phosphate cathode material LiFePO4. After passing through the battery separator and the electrolyte, they are embedded in the anode material, thus completing the charging process. The LiFePO4 that has lost Li+ becomes the delithiated product - iron phosphate FePO4.
When a lithium battery is discharging, Li+ detach from the anode material. After passing through the battery separator and the electrolyte, they return to the cathode material. When Li+ are embedded in FePO4, it changes back to LiFePO4, thus completing the discharging process.
There are various preparation processes for lithium iron phosphate, which are mainly divided into two mainstream processes: the solid-phase method and the liquid-phase method. The solid-phase method is currently the most mature and widely used synthesis method for lithium iron phosphate, while the liquid-phase method has a relatively high process difficulty. Today, the editor will introduce several preparation process methods for lithium iron phosphate:

1.Solid-phase method - Ferrous oxalate process route
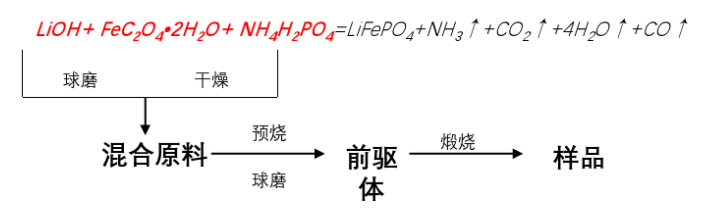
Lithium salts: Lithium carbonate (Li₂CO₃), Lithium hydroxide (LiOH), Lithium acetate (CH₃COOLi)
Iron sources: Ferrous oxalate dihydrate (FeC₂O₄·H₂O); Ferrous acetate (Fe(CH₃COO)₂)
Phosphorus sources: Ammonium dihydrogen phosphate (NH₄H₂PO₄), Diammonium hydrogen phosphate ((NH₄)₂HPO₄)
Solid-phase method - Ferrous oxalate process route: Advantages and disadvantages
Advantages: It is currently the most mainstream production route, with a simple process that is easy to industrialize.
Disadvantages:
-
The purity of ferrous oxalate is low, and there are many by-products such as FeSO₄. After being placed for a period of time, there will be more ferric iron.
-
It is very difficult to control the morphology of ferrous oxalate raw materials, resulting in poor processing performance of the finished lithium iron phosphate.
-
Since three raw materials are used, the uniformity control of the mixing materials is poor, and it is easy to oxidize, which requires high requirements for the atmosphere protection system of the sintering furnace.
-
The process route has high energy consumption, a long production cycle, the discharged ammonia is polluting, the firing rate of the product is less than 50%, and the yield is too low
2.Carbothermal reduction method - Iron phosphate process route
Lithium salts: Lithium carbonate (Li₂CO₃), Lithium hydroxide (LiOH), Lithium acetate (CH₃COOLi)
Iron and phosphorus sources: Iron phosphate tetrahydrate (FePO₄·4H₂O)
Carbon sources: Glucose, sucrose, phenolic resin, carbon black, etc.
Representative enterprises: Hunan Yuneng, Gotion High-Tech, Hubei Wanrun, Anda Technology, Longpan Technology, Fengyuan Lithium Energy, etc.
Advantages and disadvantages of the iron phosphate process route:
Advantages:
-
It can achieve one-time ball milling, one-time drying, and one-time sintering. The process is simple and energy consumption is low.
-
The firing rate is close to 70%. The raw materials are easy to be mixed uniformly, and the particles are relatively fine.
Disadvantages:
-
The performance of lithium iron phosphate materials depends severely on the quality of iron phosphate raw materials, and the iron-to-phosphorus ratio of the raw materials cannot be controlled.
-
The cost of iron phosphate alone accounts for more than 50% of the raw material cost. It is necessary to significantly reduce the cost to match its excellent electrical performance with the market price.
3.Carbothermal reduction method - Ferric oxide process route
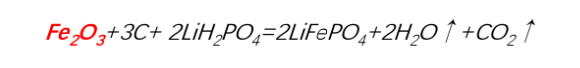
Lithium and phosphorus sources: Lithium dihydrogen phosphate (LiH₂PO₄)
Carbon sources: Glucose, sucrose, phenolic resin, carbon black, etc.
Advantages and disadvantages of the ferric oxide process route:
Advantages:
-
The raw material cost is low.
-
The morphology of lithium iron phosphate produced by this process route can be controlled, and the processing performance is the best.
-
Since two raw materials are used, the uniformity control of the mixed materials is relatively good. There is no need to consider the oxidation problem of the ferric iron source during the drying process, and the stability control is relatively good. Only one-time sintering is required. The process flow is simple and easy to control, and the yield is as high as about 80%.
Disadvantages:
-
The impurity content of ferric oxide is relatively high, and it is difficult to achieve a purity higher than 99%.
-
It is not easy to achieve an accurate stoichiometric ratio of phosphate and lithium in lithium dihydrogen phosphate.
-
The capacity is the worst, and the cost-performance ratio of the product is relatively low.
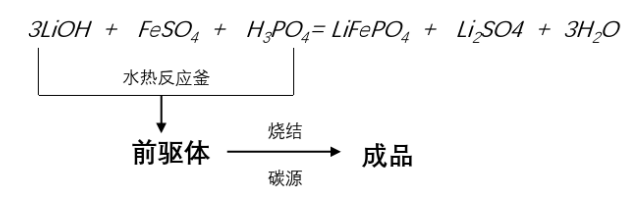
4.Liquid-phase method - Hydrothermal method
Lithium salts: Lithium carbonate (Li₂CO₃), Lithium hydroxide (LiOH), Lithium acetate (CH₃COOLi)
Iron source: Ferrous sulfate heptahydrate (FeSO₄·7H₂O)
Phosphorus source: Phosphoric acid (H₃PO₄)
Carbon sources: Glucose, sucrose, phenolic resin, carbon black, etc.